The below information is provided for use in grant proposals (i.e. Resources and Facilities section for NIH proposals).
Campus Microscopy and Imaging Facility (CMIF)
The CMIF provides high quality widefield, confocal, transmission electron microscopy, and super resolution microscopy services and support in convenient, central locations to OSU and affiliated academic institution researchers. The Office of Research (OR) supported CMIF has space with several labs on the 2nd floor of the Biomedical Research Tower (BRT) situated on the main campus. In the BRT the CMIF has 2,176 sq. ft. of lab space, which includes 702 sq. ft. of BSL2 space. This space houses 7 microscopes and individual microscope houses in its own space to reduce vibration, light, and noise disturbance during operation. In addition, there are 4 wet labs (divided into BSL1 and BSL2 space) for sample preparation and staining, and a lab for sample sectioning for TEM. The BSL2 area has a biosafety cabinet and a chemical hood and the BSL1 area has a laminar chemical hood. In addition, there is 140 sq. ft. workstation room containing 3 workstations for image analysis and post-collection image processing. CMIF also has an OR supported satellite BSL2 770 sq. ft. BSL2 imaging laboratory in the basement of the new Pelotonia Research Center, specifically designed to facilitate live cell imaging and multiple microscope modalities. The imaging facility is equipped with a biosafety cabinet and an incubator for tissue culture manipulation. The CMIF provides 24-hour access to microscopes for qualified researchers.
Services & Instrumentation
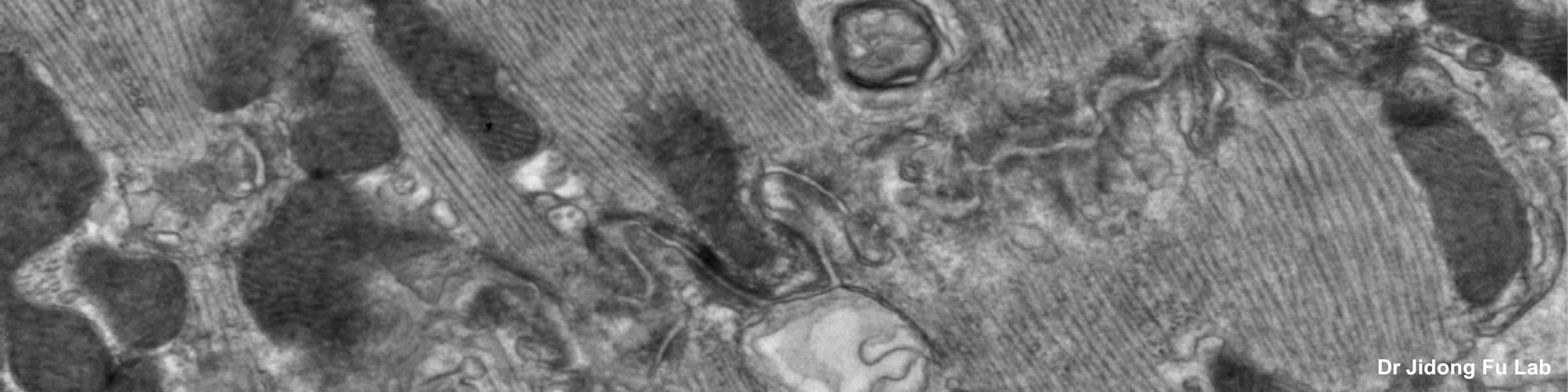
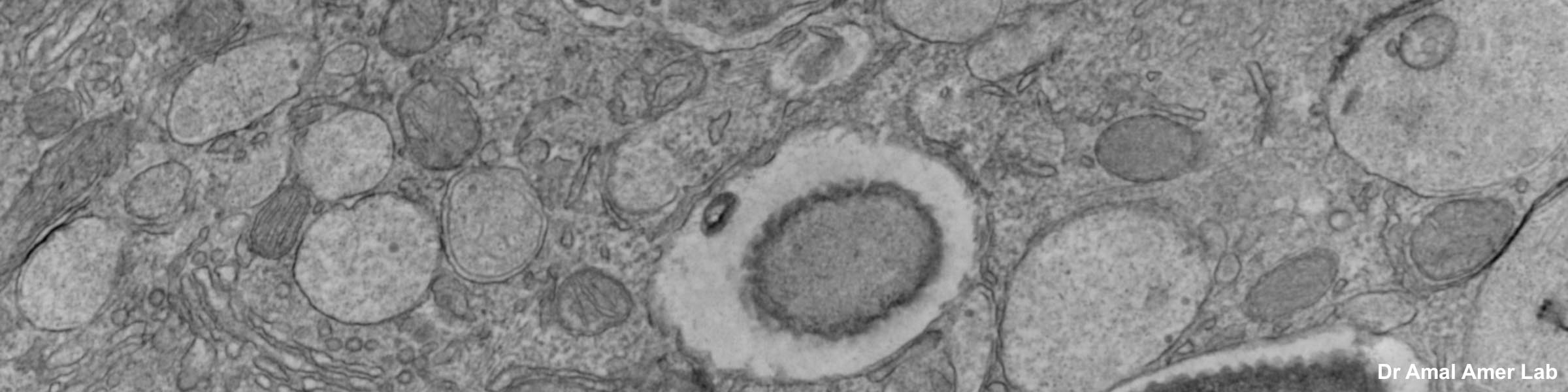
Transmission Electron Microscopy: The FEI Tecnai Spirit BioTWIN transmission electron microscope (TEM) may be used to examine a broad range of specimens, from polymers to cultured cells and tissues. The TEM is equipped with an AMT CCD camera used for standard image acquisition, and a Gatan CCD camera for capturing high resolution images. The allows general TEM imaging of biological specimens as well as screening of cryo-EM samples prior to single particle analysis (SPA) by the higher resolution Glacios CryoEM based in the OSU Center for Electron Microscopy and Analysis (CEMAS). TEM examination of ultra-thin sections supports research of cellular organelles, cell-cell interactions, viral particles, and nanostructures proposed for chemotherapeutic drug delivery. TEM can be used to determine subcellular distribution of a protein, using immunocytochemistry with gold-labeled antibodies.
Scanning Electron Microscopy: CMIF provides services to fix, critical point dry (desiccate) and sputter coat biological and hydrated materials for SEM analysis at CEMAS.
Super Resolution Microscopy: The N-SIM, N-STORM super high resolution system incorporating 2D, 3D, and TIRF modes of Structured Illumination Microscopy (SIM), traditional Total Internal Reflection (TIRF), and Stochastic Optical Reconstruction Microscopy (STORM) imaging capability. These live cell Super High Resolution light microscopy techniques enable visualization in extremely high resolution without the need for dehydration. They allow researchers to image beyond the diffraction limit of light down to 10nm resolution and single molecule imaging (TIRF-SIM). This capability enables OSU researchers to observe living processes in real-time to rapidly accelerate the progress of biomedical research. The system has two cameras for simultaneous imaging of dual fluorophores, 405, 445, 488, 514, 561, and 647 nm lasers with a compatible range of filter cubes allowing a wide range of fluorophores, and a range of objectives from 10X to 100X oil and 100X silicone. The system also has an environmental chamber for temperature and CO2 control.
Fixed Specimen Confocal Microscopy: The MSR offers three laser-scanning confocal microscopy for examination of fixed tissues or cells in cultures in our BSL area, The microscopes are housed in separate labs to minimize disturbances when imaging. Olympus FV1000 upright confocal. This system is equipped with 405, 458, 477, 488, 514, 543 and 644 nm laser lines and 4 PMT detectors and a range of dichroic mirrors for a wide range of simultaneous fluorophore imaging. There is a range of objectives from 10X to 100X oil. The system also has differential interference contrast (DIC) capability for imaging without staining and can also be used for reflected imaging and lambda scanning to detect autofluorescence in a specimen and to characterize novel fluorophores. In addition to biological specimens the upright system is ideal for surface imaging of materials for topographic analysis. Olympus FV 3000 “spectral” confocal. This inverted microscopy system has 405, 488, 561, 640 and 752 nm laser lines, 2 standard PMTs and 2 high sensitivity PMTs. The 752 nm laser is relatively unique allowing a very broad range of fluorophores with excitations from UV to near IR. A resonant scanner allows rapid scanning to reduce dwell time and laser damage on sensitive specimens. There are a range of objectives from 10X to 60X oil. Olympus FV 3000 “multi” confocal. This system is equipped with 405, 445, 488, 514, 561, 594 and 641 nm laser lines and 2 standard PMTs and 2 high sensitivity PMTs. The 7 laser lines allow unusually high flexibility with fluorophore choice. The system has a resonant scanner and a selection of objectives from 10X to 100X oil.
Light Microscopy: Zeiss Axioscope. The widefield microscope is equipped with a high sensitivity CCD camera with bright field, phase contrast, and fluorescence capabilities. High sensitivity Widefield microscopy can image extremely dim samples, which gives this technique an advantage when using fluorescence. The camera can also be used with the Olympus SZH Zoom Stereo Microscope system for low magnification imaging of whole mount or large samples not suitable for the widefield system. The stereomicroscope has an auxiliary fluorescence light source, the Nightsea viewing system, capable of illuminating samples containing green fluorescence markers (i.e. GFP).
Live Cell Imaging: The MSR provides access to cutting-edge instrumentation that can image dynamic biological processes. The Nikon A1R live cell imaging system is equipped with 402, 487, 561 and 638 nm laser lines, 2 high sensitivity PMTs, 2 high sensitivity / low noise PMTs (sensitivity up to 850 nm emission) and a PMT spectral detector and an environmental chamber for temperature and CO2 control. A high-speed piezo stage positioning system and high speed capture provides excellent high resolution temporal and spatial imaging of multiple fluorophores simultaneously. Multi-positional imaging of live or fixed cells using mosaic or ‘tiled’ imaging resulting in images much larger than the standard field of view, and allows high-throughput imaging and analysis capabilities. High-throughput imaging is particularly useful for experiments performed in 96- or 384- well plates, for applications such a drug discovery assays wherein researchers can rapidly screen the effects of various drugs or concentrations on live or fixed cells. The Nikon A1R is also equipped with the “Perfect Focus System” which allows for drift-free time-lapse imaging as short as milliseconds or as long as days. The Nikon AXR which is the 2023 upgrade release was installed in the BRT BSL2 area in September 2023. The system is equipped with 405, 488, 561 and 640 nm laser lines and 4 PMTs with tunable emission bands with 1 nm accuracy. A high speed piezo stage-positioning system (allowing XZ imaging of 600 µm thick specimens), a resonant scanner and “Perfect Focus” and stitching allows rapid 4D multi fluorophore imaging over large fields of view over extended time periods. An environmental chamber allows temperature and CO2 control. It is planned that the AXR system will be moved to the PRC as soon as the facility is completed.
Upright Olympus FV1000 Multiphoton Microscope: The multiphoton microscope (MPE) is mounted on an upright stand, specialized for imaging large or heavy specimens including live animal vital microscopy of rats and mice. The MPE can penetrate tissues up to 1000 um with an infrared laser (IR), providing a significant advantage over standard single photon confocal microscopes (maximum penetration of 30 to 50 um). The MPE is equipped with a Spectra Physics DeepSee, TiSaphire IR laser and three water immersion long working distance objectives - an IR-corrected 25X, 1.05 numerical aperture objective lens with a 2 mm working distance (W.D.), a 40X 3.3 mm W.D. and a 100X 1.5 mm W.D. This combination of deep tissue penetration and live animal imaging make the MPE a powerful tool, capable of tracking cells in live animal tissues. The system can also be used for cell damage / recovery assays. In addition the system can be used as a conventional confocal microscope with 405, 488, 594, and 643 nm laser lines and a 3 channel spectral detector, a 3 channel filter detector and a second harmonic generation (SHG) detector for label free imaging of collagen and other matrix components. The system can also be used for reflected imaging for topographical surface details and lambda scanning for autofluorescence and fluorophore characterization.
Sample Preparation: The MSR has a complete set of specialized instruments needed for preparing tissue and cells for electron microscopy. For observation with the TEM, samples can be prepared using negative staining immunocytochemistry. Cryo-sectioning and ultramicrotomy are also available for biological and some polymer samples. The critical point dryer and sputter coater are used in sample preparation for observation using high resolution SEM instruments in the CEMAS facility.
Training, Consultation and Analysis: The MSR supports extensive training opportunities for OSU and affiliated academic institution investigators. All the major instruments are available for independent use following specific training. MSR staff provide training and support on the sophisticated Imaris Image Analysis system as well as the Nikon NIS-Elements analysis suite. NIS-Elements workstations include intuitive and sophisticated 3D rendering software and a custom analysis engine which allows users to build complex analysis of images with a simple drag and drop interface. We also offer MIPAR Image Analysis package and offer basic support to users with other image analysis programs such as NIH Image-J (FIJI). The MSR offers focused workshops and individual consultation. Consultation consists of meeting with researchers to discuss experimental design and providing recommendations regarding methods of sample preparation for microscopy. If the investigator is unsure of the type of microscopy required for the project, the staff will supply advice with techniques such as immunocytochemistry and detailed protocols for a specific experiment if required. Advice is provided following imaging for various post-processing methods including understanding analysis tools (e.g. co-localization analysis, intensity measurements) and different methods of data presentation (e.g. volume rendering, maximum intensity projections). The MSR staff will also suggest content for methods sections in manuscripts.